Frankliniella schultzei (Trybom, 1910)
Thripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 8-segmented antenna, segments III and IV with forked sense cone, terminal segments VI-VIII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Head dorsal with ocellar triangle
Fig. 4: Pronotum
Fig. 5: Meso- and metanotum
Fig. 6: Fore- and hind wing, fore wing distal region
Fig. 7: Sternites V and VI
Fig. 8: Tergites VII and VIII
Fig. 9: Tergites VIII and IX
Fig. 10: Dark adult female
Fig. 11: Pale adult male
Fig. 12:
Pale adult female
Introduction and recognition
Frankliniella schultzei is an important vector of tomato spotted wilt virus, and breeds on the flowers and leaves of many plants. Both sexes fully winged; existing as two discrete color morphs, either body color brown, with pronotum, tibiae and tarsi paler, antennae brown with segments III-V yellow at base, or body color yellow with faint shadings on tergites, and antennal segments VI-VIII brown; fore wings pale with dark setae. Some authors (Bhatti et al. 2009) consider the pale and dark ones as two different species with the pale ones called as Frankliniella sulphurea, while others have synonymized both the pale and dark ones as Frankliniella schultzei. Antennae 8-segmented; segments III & IV with forked sense cone, segment VIII longer, about 1.5 the length of antennal segment VII (Fig. 1). Head wider than long; 3 pairs of ocellar setae present, pair III about as long as distance between hind ocelli and arising close together between anterior margins of hind ocelli; postocular setae pair I present, postocular setae pair IV about as long as distance between hind ocelli (Fig. 2 and 3). Pronotum with 4-5 pairs of elongate setae (1 pair anteromarginally, 1 pair anteroangularly, 2 pairs posteroangularly and 1 pair of moderately elongate posteromarginal submedian setae) (Fig. 4). Mesofurca with spinula. Metanotal median area transverse at anterior and with irregular equiangular or longitudinal reticulations on posterior half; median setae longer than lateral setae and arising at anterior margin; campaniform sensilla absent (Fig. 5). Mid and hind tarsi 2-segmented. Fore wing with 2 complete rows of veinal setae (Fig. 6). Tergites VI-VIII with paired ctenidia laterally, on VIII anterolateral to spiracle; posteromarginal comb on VIII weakly developed or absent (Fig. 8 and 9). Sternites III-VII without discal setae; median setae of sternite VII arising at or close to posterior margin (Fig. 7).
Male similar to female but smaller; tergite VIII with a few teeth laterally on posterior margin, IX with median pair of setae shorter than lateral discal setae; sternites III-VII with broadly transverse glandular area.
Second instar larva white with antennal segments pale grey and tergite X with distinct grey band on posterior half; posterior margin of tergite IX with small teeth; spiracles on VIII small.
Taxonomic identity
Species
Frankliniella schultzei (Trybom, 1910)
Taxonomic history
Frankliniella ipomoeae Moulton, 1948
Frankliniella nigra Moulton, 1948
Frankliniella clitoriae Moulton, 1940
Frankliniella pembertoni Moulton, 1940
Frankliniella favoniana Priesner, 1938
Frankliniella lycopersici Andrewartha, 1937
Frankliniella nana Priesner, 1936
Frankliniella kellyana Kelly & Mayne, 1934
Frankliniella paucispinosa Moulton, 1933
Frankliniella insularis Morison, 1930
Parafrankliniella nigripes Girault, 1928
Frankliniella aeschyli Girault, 1927
Frankliniella africana Bagnall, 1926
Frankliniella anglicana Bagnall, 1926
Frankliniella interocellaris Karny, 1925
Frankliniella tabacicola Karny, 1925
Frankliniella dampfi Priesner, 1923
Frankliniella persetosa Karny, 1922
Frankliniella trybomi Karny, 1920
Frankliniella pallida Karny, 1920
Frankliniella delicatula Bagnall, 1919
Frankliniella sulphurea Schmutz, 1913
Physopus schultzei Trybom, 1910
Common name
Common blossom thrips
Cotton bud thrips
Tomato thrips
Yellow flower thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Frankliniella Karny, 1910
Genus description
The genus Frankliniella Karny, 1910
This genus is mainly known from the New World and contains about 230 species, many of them from the Neotropics (Mound & Marullo 1996, Cavalleri & Mound 2012). Some species are widely known as crop pests - Frankliniella occidentalis, Frankliniella intonsa, Frankliniella schultzei (all of them vectors of tospoviruses) and Frankliniella williamsi. The members in this genus are sometimes quite difficult to separate from one another and the classification has been in flux with many species later synonymized in association with color variations. They mostly have 3 pairs of well developed ocellar setae, 8-segmented antennae with segments III and IV having forked sense cones, usually 4-5 pairs of elongate pronotal setae (1 pair anteromarginally, 1 pair anteroangularly, 2 pairs posteroangularly, and 1 pair of moderately elongate posteromarginal submedian setae S2 which are longer than median seta S1), metanotal median setae arising at anterior margin, when present wings with complete rows of setae on the wing veins, paired ctenidia laterally on tergites V-VIII with those on VIII anterolateral to the spiracles, no discal setae on sternites, and the males are generally smaller and paler than the females (Mound & Marullo 1996; Stannard 1968).
Species description
Typical key character states of Frankliniella schultzei
Coloration and body sculpture
Body color: mainly brown to dark brown or mainly pale to yellow, or with some darker markings
Surface of head, pronotum and fore legs: without obvious or with weakly reticulate sculpture
Antennae
Form of sense cones on antennal segments III and IV: emergent and forked on segments III and IV
Number of antennal segments: 8
Antennal segment I: without any setae on dorsal apical margin
Antennal segment II: without an exceptionally long seta at the inner apex
Antennal segment II shape: symmetric
Antennal segment III shape: symmetric
Shape of pedicel on antennal segment III: simple
Length of antennal segment III and IV: antennal segment III similar in length to segment IV
Forked sense cone on antennal segment IV: scarcely extending beyond base of segment V
Antennal segment IV and V: without a hyaline ring near the base
Antennal segment VI bears: not a remarkably dagger-shaped sensorium
Antennal segment VIII length: longer, about 1.5 the length of antennal segment VII
Head
Distance between bases of ocellar setae III: same or less than width of first ocellus
Head: not prolonged in front of compound eyes
Pair of major postocular seta: longer than other postoculars and as long as distance between hind ocelli
Ocellar setae I: present
Length of ocellar setae II: shorter than setae III
Ocellar setae III: arising on anterior margin of, or between hind ocelli
Ocelli: present
Ocellar setae III length: about as long as distance between hind ocelli
Length of postocular setae: not alternating short and long setae
Number of ocellar setae: 3
Prothorax
Number of pairs of anteromarginal minor setae: 2-3
Number of pairs of long anteroangular setae: 1-2
Number of pairs of long posteroangular setae: 2
Number of pairs of elongate pronotal setae: 4-5
Number of pairs of posteromarginal minor setae: 4-5
Pronotal blotch or internal apodeme: absent
Pronotum shape: broadly rectangular
Pronotum posteromarginal/posteroangular setae: S2 longer than S3, not equal in length
Mesothorax
Mesosternal furca: with median spinula
Metathorax
Metanotal campaniform sensilla: absent
Metanotal median setae: S1 at anterior margin
Metanotum with dominant sculptured triangle medially: absent
Metasternal furca: without spinula
Shape of metathoracic furca: transverse, V-shaped
Metanotal median setae length: longer than lateral metanotal setae
Sculpture of metanotum median area: transverse at anterior, but equiangular reticulations on posterior half or irregular longitudinal or equiangular reticulations on posterior half
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous)
Fringe cilia arising: from sockets
Fore wing veins: present
Fore- and hind wing surface: covered with microtrichia
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with setae and cilia but cilia longer than setae
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: distinct from costal vein
Fore wing first vein setal row: complete, with setae closely and uniformly spaced
Fore wing second vein setal row: complete, setae uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fringe cilia on posterior margin near apex: distinctly wavy (undulated)
Length of fore wing costal setae at middle of wing: longer than half of median wing width
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wing extreme apex color: pale
Fore wings: uniformly pale or weakly shaded
Legs
Fore tibia: not prolonged around fore tarsus
Mid and hind tarsi: with two segments
Color of fore tarsi: brown, or pale or yellow, sometimes apical shaded or brown
Abdomen
Pleurotergites: not covered in microtrichia
Sternite II: with marginal setae but no discal setae
Sternites IV, V and VI: with marginal setae but no discal setae
Sternite VII: with marginal setae but no discal setae
Surface of lateral thirds of abdominal tergites: without regular rows of fine microtrichia
Tergites II to VII median setal pair: no more than 0.3 as long as median length of tergite
Craspedum on tergites IV to VI: absent
Tergites IV and V median setal pair: shorter than distance between their bases
Tergites V to VII: with ctenidia laterally
Craspedum on tergite VIII: without craspedum medially and toothlike microtrichia laterally
Tergite VIII ctenidia: anterolateral to spiracle
Tergite VIII shape of posteromarginal microtrichia: short and irregular in length
Tergite VIII posteromarginal comb of microtrichia: absent or present and complete medially or present laterally, incomplete medially
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
Frankliniella schultzei is exceptional within the genus because ocellar setae III arising very close together between anterior margins of hind ocelli (Frankliniella occidentalis, Frankliniella borinquen and Frankliniella williamsi with ocellar setae III arising on anterior margins of or just within anterior margins of ocellar triangle), metanotal campaniform sensilla are absent (in other species present), and tergite VIII has the posteromarginal comb of microtrichia weakly developed or complete absent (in Frankliniella borinquen and Frankliniella occidentalis the comb on tergite VIII is complete, in Frankliniella borinquen with short microtrichia arising from triangular bases; in Frankliniella occidentalis with short or long microtrichia on broadly triangular bases; Frankliniella williamsi with a comb of long and fine, closely spaced microtrichia on broadly triangular bases). Frankliniella schultzei as well as Frankliniella occidentalis and Frankliniella williamsi with simple pedicel of antennal segment III and antennal segment VIII longer than segment VII (only Frankliniella borinquen with a pedicel of antennal segment III swollen, with edged ring surmounted by a distinctive swelling and a slightly flared collar, and antennal segment VIII equal in length to or shorter than segment VII). In Frankliniella schultzei and Frankliniella williamsi ocellar setae III on head are about as long as distance between hind ocelli (in Frankliniella occidentalis ocellar setae III are about as long as distance between external margins of hind ocelli, and in Frankliniella borinquen as long as distance between midpoints of hind ocelli). In Frankliniella borinquen as well as Frankliniella schultzei the length of postocular setae IV are about as long as distance between hind ocelli (in Frankliniella occidentalis postocular setae IV are longer and in Frankliniella williamsi distinctly shorter than distance between hind ocelli). Compared to Frankliniella williamsi which has 1 or 2 median discal setae in addition to marginal setae on sternite II, in other species of Frankliniella sternite II without median discal setae.
Species of the genus Frankliniella are similar to species of Thrips, Stenchaetothrips, Microcephalothrips abdominalis, Larothrips dentipes and Fulmekiola serrata, in having tergites V-VIII which bear a pair of ctenidia laterally, but in those species ctenidia of the tergite VIII lie posteromedial to the spiracle, whereas species of Frankliniella have ctenidia on tergite VIII which lie anterolateral to the spiracle.
Biology
Life history
Life cycle usually takes about 2-5 weeks (Hill 1983); with eggs hatch in 5-8 days, nymphs undergo 3 instars in 8-10 days, pupation lasts 4-7 days (Gahukar 2004). On tomato the embryonic period lasts 4.3 d; the 1st instar 2.5 d; the 2nd instar 2.5 d; prepupa 1.2 d; pupa 2.1 d; duration of the immature phase 8.3 d; male longevity lasts 13.1 and female longevity 13.6 d. The life cycle duration takes 12.6 d (Pinent and Carvalho, 1998).
Host plants
Polyphagous and flower feeding; compositae crops and weeds.
Crops: African nightshade, African spiderplant, amaranth, babycorn, beans (broad bean, common bean, cowpea, French bean, garden pea, hyacinth bean, Lima bean), beet root, brocolli, cabbage, capsicum, cassava, chillies, coriander, cotton, courgette, cucurbit, eggplant, green gram, groundnut, leek, kale, maize, okra, onion, potato, pumpkin, red gram, sorghum, sunflower, sweet potato, thorn apple, tobacco, tomato, watermelon, wheat.
Weeds: Achyranthes aspera, Ajuga remota, Bidens pilosa, Chenopodium sp., Conyza bonariensis, Crotolaria sp., Datura suaveolens, Dyschoriste radicans, Erlangea calycina, Galinsoga parviflora, Guizotia scabra, Hermannia oliveri,Lantana camara, Malvaviscus grandiflorus, Melhania velutina, Nicandra physalodes, Sesbania sesban, Solanum incanum, Sonchus oleraceus, Tagetes minuta, Tithonia diversifolia.
Vector capacity
Capsicum chlorosis virus (CaCV)
Chrysanthemum stem necrosis virus(CSNV)
Groundnut ringspot virus (GRSV)
Impatiens necrotic spot virus (INSV)
Tomato chlorotic spot virus TCSV)
Tomato spotted wilt virus (TSWV)
Also known to carry spores, mildews, rusts and other fungi.
The dark forms of Franklinella schultzei are competent vectors of the above five tospoviruses, however the pale forms are not competent virus vectors (Sakimura 1969; Whitfield et al. 2005, Reitz et al. 2011).
Damage and symptoms
The species breeds on the flowers and leaves and damages young leaves within the meristematic bud. They rasp the cells off the upper surface of young leaves, while they are still in the bud (Hill 1983). Silvery scars appear on the upper surface of leaves and those leaves distorted. Adults prefer young leaves and flowers, nymphs leaf buds (Gahukar 2004).
Detection and control strategies
Frankliniella schultzei are attracted to both yellow and blue sticky traps, which could be used for effective monitoring of their dynamics in the field. Further their attraction to coloured sticky traps is enhanced by flower based kairomones (Muvea, 2011).
Intercropping of cowpea with pearl millet under different configurations significantly reduced the abundance of Frankliniella schultzei (Gahukar 1989). Frankliniella schultzei was highly attracted to Irish Potato intercropped with French beans (Nyasani et al. 2012). In Senegal, cowpea and wild groundnut, Arachis diogoi, showed tolerance to pest attack (Gahukar 1987). The Australian phytoseiid mite Typhlodromips montdorensis (Acari: Phytoseiidae) has a daily consumption rate of 10.63 first-instar thrips larvae of Frankliniella schultzei (Steiner et al. 2003).
Additional notes
It is also an effective predator of mites on some crops such as cotton.
Biogeography
Tropical to subtropical and southern Mediterranean, Africa, Asia, Australia, New Zealand, Central and South America, North America. Angola, Benin, Botswana, Burkina Faso, Cameroon, Cape Verde, Central African Republic, Chad, Congo (Brazzaville), Egypt (Maadi), Ethiopia, Gabon, Gambia (Abuko, Kombo), Ghana (Aburi), Kenya, Libya, Madagascar, Maurititius, Morocco, Niger, Nigeria, Namibia, Senegal, Somalia, South Africa (Eastern Cape: Somerset East; Western Cape: Somerset West; Prince Albert Road), Sudan, Tanzania, Tunisia, Togo (Wonugba), Uganda, Zambia, Zimbabwe.
African countries where Frankliniella schultzei has been reported
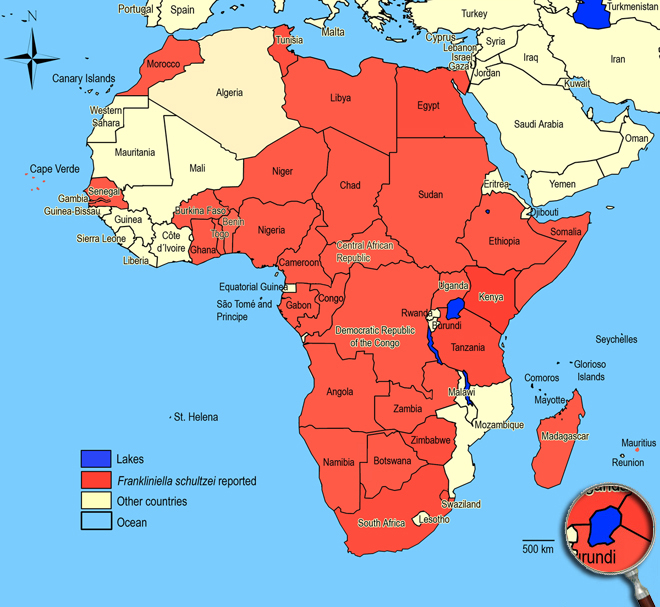
Occurence of Frankliniella schultzei in East Africa
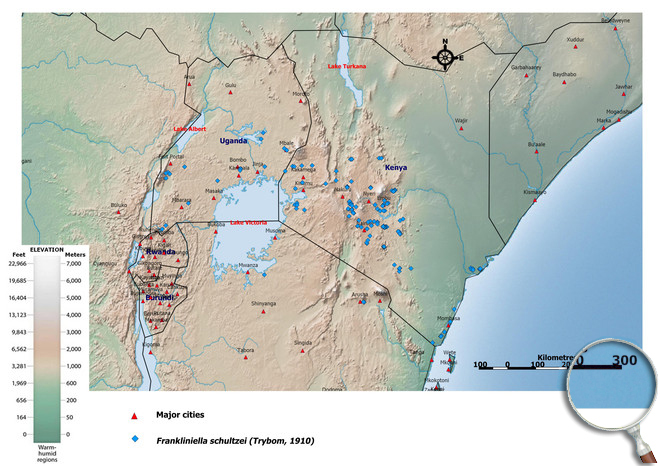
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Frankliniella schultzei in parts of East Africa.

Bibliography
Amin PW & Palmer JM (1985). Identification of groundnut Thysanoptera. Tropical Pest Management. 31 (4): 286-291
Ananthakrishnan TN (1971). Thrips (Thysanoptera) in agriculture, horticulture & forestry - diagnosis, bionomics & control. Journal of Scientific and Industrial Research. 30 (3): 113-146
Bagnall RS (1919). Brief descriptions of new Thysanoptera - X . Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 9) 4: 253-277
Bagnall RS (1926). Brief descriptions of new Thysanoptera - XV. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 9) 18: 98-114
Bhatti JS (2006). The classification of Terebrantia (Insecta) into families. Oriental Insects. 40 (1): 339-375
Cavalleri A & Mound LA (2012). Toward the identification of Frankliniella species in Brazil (Thysanoptera, Thripidae). Zootaxa 3270: 1-30
de Borbon CM (2007). A key for the identification of second instar larvae of some common thrips (Thysanoptera: Thripidae). Revista De la Facultad de Ciencias Agrarias, Mendoza, Argentina. 39 (1): 69-81
Gahukar RT (1987). Programme d´entomologie: synthèse et activitiés (1981-87) et recommendations. Rapport Preparé pour le Gouvernement du Sénégal, FAO, Rome
Gahukar RT (1989). Pest and disease incidence in pearl millet under different plant density and intercropping patterns. Agriculture, Ecosystems and Environment. 26: 69-74
Gahukar RT (2004). Bionomics and management of major thrips species on agricultural crops in Africa. Outlook on Agriculture. 33 (3): 191-199
Hill D (1983). Agricultural insect pests of the tropics and their control, (2nd edition). Cambridge University Press, Cambridge, 746 pp
Karny H (1912). Revision der von Serville aufgestellten Thysanopteren-Genera. Zoologische Annalen. 4 (4): 322-344
Karny H (1925). On some tropical Thysanoptera. Bulletin of Entomological Research. 16 (2): 125-142
Kirk WDJ (1987). A key to the larvae of some common Australian flower thrips (Insecta: Thysanoptera), with a host-plant survey. Australian Journal of Zoology. 35: 173-185
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp
Lewis T (1997). Thrips as crop pests. CAB International, Wallingford, 740 pp
Moore ES & Anderssen EE (1939). Notes on plant virus diseases in South Africa. Science Bulletin, No. 182. Department of Agriculture, Union of South Africa, 36 pp
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Moulton D (1940). Thysanoptera from New Guinea and New Britain. Occasional Papers of Bernice P. Bishop Museum (HBS-Bishopmuseum), Honolulu, Hawaii. 15 (24): 243-270
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainsville, 487 pp
Muvea AM (2011). The potential of coloured sticky traps with kairomonal attractants (LUREM-TR) in management of thrips on Tomato and French beans. Unpublished thesis, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya, 107 pp
Nickle DA (2003). A checklist of commonly intercepted thrips (Thysanoptera) from Europe, the Mediterranean, and Africa at U.S. ports-of-entry (1983-1999). Part I. Key to genera. Proceedings of the Entomological Society of Washington. 105 (1): 80-99
Nickle DA (2004). Commonly intercepted thrips (Thysanoptera) from Europe, the Mediterranean, and Africa at U.S. ports-of-entry. Part II. Frankliniella Karny and Iridothrips Priesner (Thripidae). Proceedings of the Entomological Society of Washington. 106 (2): 438-452
Nyasani JO, Meyhöfer R, Subramanian S & Poehling H.-M (2012). Effect of intercrops on thrips species composition and population abundance on French beans in Kenya. Entomologia Experimentalis et Applicata 142: 236-246
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Pitkin BR & Mound LA (1973). A catalogue of West African Thysanoptera. Bulletin de ľInstitut Fondamental ďAfrique Noire, Série A. 35 (2): 407-449
Pinent SMJ & Carvalho GS (1998). Biology of Frankliniella schultzei (Trybom) (Thysanoptera: Thripidae) in tomatoes. Anals da Sociedade Entomologica do Brasil 27 (4): 519 - 524
Priesner H (1925). Neue Thysanopteren. Deutsche Entomologische Zeitschrift. 1925: 13-28
Priesner H (1936). On some further new Thysanoptera from the Sudan. Bulletin de la Société Royale Entomologique ďEgypte. 20: 83-104
Priesner H (1938). Thysanopterologica VI. Konowia. 17: 29-35
Sakimura K (1969). A comment on the color forms of Frankliniella schultzei (Thysanoptera: Thripidae) in relation to transmission of the tomato spotted wilt virus. Pacific Insects, 11, 761-762
Stannard LJ (1968). The thrips, or Thysanoptera, of Illinois. Illinois Natural History Survey Bulletin. 29 (4): 214-552
Steiner MY, Goodwin S, Wellham TM, Barchia IM & Spohr LJ (2003). Biological studies of the Australian predatory mite Typhlodromips montdorensis (Schicha) (Acari: Phytoseiidae), a potential biocontrol agent for western flower thrips, Frankliniella occidentalis. (Pergande) (Thysanoptera: Thripidae). Australian Journal of Entomology. 42: 124-130
Timm AE, Stiller M & Frey JE (2008). A molecular identification key for economically important thrips species (Thysanoptera: Thripidae) in southern Africa. African Entomology. 16 (1): 68-75
Trybom F (1910). Physopoda. In Schultze L [ed.], Zoologische und anthropologische Ergebnisse einer Forschungreise im westlichen und zentralen Südafrika ausgeführt in den Jahren 1903-1905 mit Unterstützung der kgl. Preussischen Akademie der Wissenschaften zu Berlin. Denkschriften der Medizinisch-naturwissenschaflichen Gesellschaft zu Jena. 16 (4): 147-174
Whitfield AE, Ullman DE & German TL (2005). Tospovirus thrips interactions. Annual Review of Phytopathology, 43, 459-489
zur Strassen R (1958). Notes on Thysanoptera of tropical Africa and St. Helena. Journal of the Entomological Society of Southern Africa. 21 (1): 69-79
zur Strassen R (1960). Catalogue of the known species of South African Thysanoptera. Journal of the Entomological Society of Southern Africa. 23 (2): 321-367
zur Strassen R (1969). Neue Angaben zur Thysanopteren-Fauna (Insecta, Thysanoptera) der Kanarischen Inseln. Commentationes Biologicae. 31 (5): 1-74
zur Strassen R (1983). Thysanopterologische Notizen (6) (Insecta: Thysanoptera). Senckenbergiana Biologica. 63 (3-4): 191-209
zur Strassen R (1993). Chorologische, phänologische und taxonomische Studien an Terebrantia der Kapverden (Insecta: Thysanoptera). Courier Forschunginstitut Senckenberg. 159: 335-380
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California
















